A leitmotiv within all our research lines is the use of bulky ligands. Among those, our group has been part of an effort to use crowded phosphines that contain a terphenyl (2,6-diarylphenyl) fragment. Over the last few years we have demonstrated their potential to stabilize otherwise elusive organometallic frameworks. Beyond terphenyl phosphines we are also developing other highly congested ligands, as well as re-investigating others that have been mostly overlook. An example of the latter is the cavity-shaped and extremely bulky tris-2-(4,4′-di-tert-butylbiphenylyl) phosphine, which allowed us to isolate the first dicoordinate Au(I) ethylene adduct and study its role in catalytic olefin hydroamination (see Figure).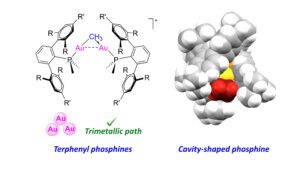
See for example:
- Navarro, M.*; Alférez, M. G.; de Sousa, M.; Miranda-Pizarro, J.; Campos, J.* Dicoordinate Au(I)–Ethylene Complexes as Hydroamination Catalysts, ACS Catal. 2022, DOI:10.1021/acscatal.1c05823
- Navarro, M.; Miranda-Pizarro, J.; Moreno, J.J.; Navarro-Gilabert, C.; Fernández, I.*; Campos, J.* A dicoordinate gold(I)–ethylene complex, Chem. Commun. 2021, 57, 9280. [Highlighted in Chemistry Views, 25 August 2021]
- Miranda-Pizarro, J.; Luo, Z.; Moreno, J. J.; Dickie, D. A.; Campos, J.*; Gunnoe, T. B.* Reductive C–C coupling from Molecular Au(I) Hydrocarbyl Complexes: A Mechanistic Study, J. Am. Chem. Soc. 2021, 143, 2509.
- Moreno, J. J.; Espada, M. F.; Campos, J.; López-Serrano, J.; Macgregor, S. A.; Carmona, E. Base-Promoted, Remote C-H Activation at a Cationic (η5-C5Me5)Ir(III) Center Involving Reversible C-C Bond Formation of Bound C5Me5, J. Am. Chem. Soc. 2019, 141, 2205.
